| Brief communication | Peer reviewed |
Cite as: Woon SY, Barton MD, Vanniasinkam T. Effect of plasma transfer on survival rates of low-birth-weight neonatal piglets. J Swine Health Prod. 2014;22(4):197–200.
Also available as a PDF.
SummaryPlasma transfer was evaluated as a strategy to enhance survival rates of low-birth-weight piglets. Plasma administration did not significantly affect weight gain or survival rates, demonstrating that plasma transfer alone cannot be used to improve survival rates of low-birth-weight piglets. | ResumenSe evaluó la transferencia de plasma como estrategia para mejorar el porcentaje de supervivencia de lechones con peso bajo al nacimiento. La administración de plasma no afectó significativamente la ganancia de peso o el porcentaje de supervivencia, demostrando que la transferencia de plasma por sí sola no puede utilizarse para mejorar los índices de supervivencia de lechones con bajo peso al nacimiento. | ResuméLe transfert de plasma fut évalué comme stratégie pour augmenter les taux de survie de porcelets de faible poids à la naissance. L’administration de plasma n’affecta pas de manière significative le gain de poids ou les taux de survie, démontrant ainsi que le seul transfert de plasma ne peut être utilisé pour améliorer les taux de survie de porcelets de faible poids à la naissance. |
Keywords: swine, piglet mortality, plasma transfer, immunoglobulin transfer, plasma therapy
Search the AASV web site
for pages with similar keywords.
Received: May 31, 2013
Accepted: November 20, 2013
Neonatal piglet mortality, especially in low-birth-weight (LBW) piglets, remains a major issue in pig farming. Despite advancement in pig production management over the years, up to 24% losses of newborn piglets are encountered by pig producers.1-3 Causes of piglet mortality range from crushing by the sow to disease and poor viability.4,5 The pain and distress that these piglets undergo prior to death remains an important animal-welfare issue.
High demand for pork in the consumer market has caused an increase in sow productivity, with sows producing big litters selected for breeding by farmers.6 A big litter size is often associated with more low-birth-weight piglets and higher rates of mortality, compared with the industry average.2,7,8 Small piglets have inadequate energy stores and may also have limited access to colostrum.8-10 These piglets also often have inadequate maternal antibodies to protect them against common pathogens such as Escherichia coli, with higher pre-weaning mortality from infections being reported in these piglets.9,11,12
Many strategies to improve immune status as well as survival of LBW piglets have been evaluated over the years,13-16 including plasma or serum transfer. Overall, studies involving plasma or serum transfer in pigs15,17 and other species, eg, horses,18-20 have shown variable results. A pilot study undertaken at a swine-production facility (5600 sows in a continuous-flow production system), involving administration of plasma to LBW piglets, showed that pre-weaning mortality was 4% lower in the plasma-treated piglets (unpublished data). However, as the sample size was small, the results of the pilot study were not statistically significant. The current study was performed at the same swine-production facility to determine if plasma transfer could be used effectively in commercial farms as a strategy to improve the overall health of LBW piglets and reduce mortality.
Materials and methods
This study was approved by the Charles Sturt University Animal Care and Ethics Committee and the swine production facility’s animal ethics committee.
A total of 612 piglets (body weight 0.8 to 1.3 kg) from 212 dams (Large White × Landrace) were randomly allocated in equal numbers to one control and two treatment groups (piglet ID numbers drawn from a container). Piglets in this weight range were chosen on the basis of data obtained from a pilot study showing that approximately 40% preweaning mortality occurred in these piglets (unpublished data).
Fostering is standard practice in Australian pig farms to help manage big litters; therefore, fostering was undertaken in keeping with standard practice on the study farm. A total of 68 foster sows were used in this study. Not all piglets assigned to these foster sows were enrolled in the study. Piglets were fostered 6 to 24 hours after birth to a foster sow and remained with that dam for the duration of the study. The litter size per foster dam ranged from nine to 14 (average approximately 11 piglets), with the number of piglets per foster dam dependant on teat availability. The birth dams and foster dams were of parities two to four: information on parity of individual sows was not made available to the researchers.
The following management practices were followed on the study farm, which comprised 5600 sows in a continuous-flow production system. Sows were group housed until 110 days of gestation, when they were housed singly in farrowing crates. As this study was conducted on a commercial farm, data on pre-weaning mortality, total births, and other production parameters were not available to the researchers. All piglets and pigs were housed in a conventional, naturally ventilated barn thermostatically controlled at 28°C. Piglets and pigs were given the standard vaccinations used on the study farm, fed age-appropriate diets, and allowed unrestricted access to water. Piglets remained with the sow from birth until weaning at 28 days.
Day 0 was the day of birth and Day 1 the first day of treatment. Piglets in the treatment groups received either two doses of plasma (10 mL on two separate occasions, Day 1 and Day 3) or one dose of plasma (10 mL on one occasion, Day 1) by intramuscular injection in four sites on the neck and hind legs, ie, 2.5 mL per site. On Day 1 and Day 3, control piglets received intramuscular injections of 10 mL of Hartmann’s solution (also known as compound sodium lactate and similar to lactated Ringer’s solution) as described for the two treatment groups.
Plasma was obtained from Large White × Landrace donor sows from the same farm and processed by ACE Laboratory services, Bendigo, Australia, using a standard commercial membrane filtration method, following guidelines approved under the Australian Pesticides and Veterinary Medicines Authority. Briefly, plasma was centrifuged and then filtered using a pressurized filtration method through three filters (5 to 0.2 microns). Immunoglobulin G (IgG) concentrations in the pooled plasma before and after processing (46.3 mg per mL and 32.4 mg per mL, respectively) were measured using a commercially available kit (Pig IgG ELISA Quantitation Set; Bethyl Laboratories, Montgomery, Texas).
Piglets were physically examined to assess their condition and weighed on Days 7, 14, and 21. Blood samples were obtained from piglets on Days 0, 2, and 6, and ELISA for detection of porcine IgG was performed on serum using a kit (Pig IgG ELISA Quantitation Set; Bethyl Laboratories). The ELISA kit was validated for quantification of porcine IgG using immunoglobulin standards supplied by the manufacturer. Mortality in the three groups was recorded by one of the researchers daily after piglets were enrolled in the study.
All data were analysed using a linear mixed model in ASReml-R (VSN International, Hemel Hempstead, United Kingdom). This analysis was undertaken to account for variability caused by the effects of various factors (eg, foster sow and birth sow). In order to determine the effect of plasma treatment on IgG concentrations in piglets over time, a linear mixed model using restricted maximum likelihood was used. Birth weight, day of treatment, and treatment type were included as fixed terms in the model. Foster sow and birth sow were fitted as random terms. The effect of the plasma treatment on weekly weight gain was analysed using a linear mixed model with birth weight, treatment type, and day of treatment included as fixed terms. Foster sow and birth sow were fitted as random terms. Effect of plasma treatment on total weight gain was analysed with a linear mixed model with birth weight and treatment type included as fixed terms. Foster sow and birth sow were fitted as random terms. Differences were considered significant at P < .05.
Results
The plasma administered in this study, when processed, had an estimated 30% loss of total immunoglobulins. The IgG concentration in the serum of the piglets was in the range of 17.1 to 21.6 mg per mL when measured at Day 2, and slowly declined to 14.1 to 18.4 mg per mL by Day 6 in both treatment groups and in the control group (Figure 1). Serum IgG concentrations did not differ significantly between the two treatment groups and the control group.
Figure 1: Effect of plasma transfer on the survival of low-birth-weight piglets was determined. Piglets were randomly allocated into two treatment groups and one control group, with 204 piglets per group. Piglets in the treatment groups were administered plasma obtained from donor sows on the same farm (10 mL plasma intramuscularly in four sites per treatment) on Day 1 or Day 1 and Day 3, with Day 0 defined as the day of birth. The control group was similarly injected with 10 mL Hartmann’s solution on days 1 and 3. Serum samples were obtained from the piglets on days 0, 2, and 6. Total IgG concentrations, measured using a commercially available kit (Pig IgG ELISA Quantitation Set; Bethyl Laboratories, Montgomery, Texas), did not differ significantly between groups (P > .05).
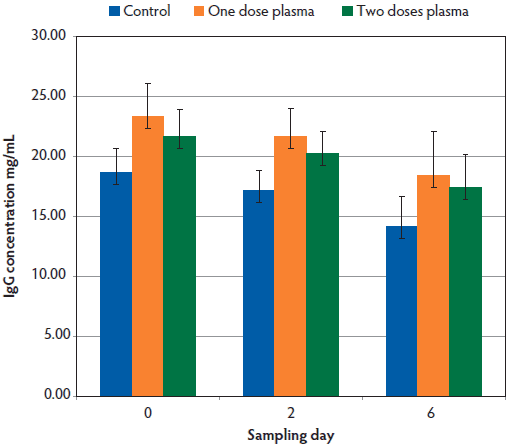
Similarly, there was no statistically significant difference in weight gain between the two treatment groups and the control group. When piglets were recruited into the study, they were all of low birth weight, and visually there were no differences between the piglets in the three groups. The average daily weight gain was 163.5 g in the group that received one dose of plasma, 164.0 g in the group that received two doses of plasma, and 163.9 g in the control group. The analysis of individual weights of the piglets showed that total weight gain in piglets over the 3-week period was influenced by the birth weight of the piglet (P < .05), with those that were heavier at birth gaining more weight over this period (data not shown). The birth sow had an influence on the IgG concentrations in the piglet serum at all sampling points, with piglets from seven birth sows having significantly higher IgG concentrations than the others (P < .05).
The highest mortality in piglets was recorded in those that received two doses of plasma, with most deaths occurring on Days 0, 1, and 2. By Day 21, 32.5% of piglets that received two doses of plasma, 26.6% of piglets that received one dose of plasma, and 26.5% of control piglets had died. The causes of death were poor vitality (41%), crushing by the sow (27%), and diarrhea (22%). Diarrhea was diagnosed by observation of watery stools; the causative organism of the condition was not determined.
Discussion
The results of this study showed that administration of porcine plasma did not significantly affect LBW piglet weight gain or survival. Piglets that were heavier at birth were more robust and demonstrated better growth performance than piglets of lower birth weight. Similar results have been reported by other investigators.2,7 The birth sow was an important factor in determining IgG concentrations in the serum of piglets. As IgG is the predominant immunoglobulin in colostrum, the amount of IgG taken up by the piglets depends upon the quantity or quality of colostrum obtained from the birth sow within 24 hours of birth, and this would be important in providing immunity from common infections.1,21 Researchers have shown that the concentration of IgG in colostrum may be variable even within sows from the same unit.22 Furthermore, IgG concentrations in colostrum also vary with the position of the teats.22 In one report, the colostrum from cranial teats, for example, had higher IgG concentrations than colostrum from the caudal teats.23 The birth sow would also have contributed to the first weekly weight gain of the piglets, as body fat reserves of newborn piglets are deposited in the last month of gestation.24 These data underscore the importance of the birth sow in determining serum IgG concentrations of LBW piglets.
Highest mortality in this study occurred in the group of piglets that received two doses of plasma. The reason for this could not be determined. Another important finding of this study was that most deaths in LBW piglets (in all groups) were caused by poor vitality, possibly due to inadequate consumption of colostrum. It is possible that the full fostering practice adopted in this study may have contributed to inadequate consumption of colostrum. Fostering is widely practised in piggeries across Australia, and the results of this study show that this practice may seriously disadvantage LBW piglets. Teat order is established by 24 hours following birth, and therefore fostering would cause increased fighting among the piglets, with smaller LBW piglets forced to suckle from non-productive teats.6 It would be useful to explore the impact of birth and foster sows on plasma transfer in neonatal piglets, in particular, the effect of birth and foster-sow parity on IgG concentrations.
The membrane filtration method used to process the plasma may have contributed to the loss of immunoglobulins observed during plasma processing in this study. If plasma transfer is to be undertaken successfully, it is important to examine other technologies for processing plasma with a minimal loss of immunoglobulins. In addition, strategies such as concentrating plasma by spray drying would enable larger amounts of antibodies to be delivered in smaller volumes to the neonatal piglets. On the basis of the results of this study, it appears plasma transfer alone is not sufficient to improve survival of LBW piglets. In future studies, it would be worthwhile to evaluate the efficacy of plasma transfer in conjunction with other farming practices, such as minimal fostering, in order to improve LBW piglet health and survival.
Implications
• Under the conditions of this study, plasma transfer alone does not improve survival of LBW piglets
• Further studies are required to determine if plasma transfer used in conjunction with other farming practices can improve overall welfare and survival of LBW piglets.
Acknowledgements
The authors would like to thank the staff on the farm in which this study was conducted for help with obtaining blood samples from the piglets, the staff at ACE Laboratory Services for processing the plasma used in this study, and Sharon Nielsen (Charles Sturt University) for help with the statistical analysis of data. This study was funded by the Australian Pork Cooperative Research Centres.
Conflict of interest
None declared.
References
1. Devillers N, Le Dividich J, Prunier A. Influence of colostrum intake on piglet survival and immunity. Animal. 2011;5:1605–1612. Available at: http://dx.doi: 10.1017/S175173111100067X. Accessed 15 April 2014.
2. Pedersen LJ, Jorgensen E, Heiskanen T, Damm BI. Early piglet mortality in loose-housed sows related to sow and piglet behaviour and to the progress of parturition. Appl Anim Behav Sci. 2006; 96:215–232. doi: 10.1016/j.applanim.2005.06.016.
3. Weber R, Keil NM, Fehr M, Horat R. Piglet mortality on farms using farrowing systems with or without crates. Anim Welfare. 2007;16:277–279.
4. Baxter EM, Jarvis S, Palarea-Albaladejo J, Edwards SA. The weaker sex? The propensity for male-biased piglet mortality. PLoS One. 2012;7:e30318. doi: 10.1371/journal.pone.0030318.
5. Roehe R, Shrestha NP, Mekkawy W, Baxter EM, Knap PW, Smurthwaite KM, Jarvis S, Lawrence AB, Edwards SA. Genetic parameters of piglet survival and birth weight from a two-generation crossbreeding experiment under outdoor conditions designed to disentangle direct and maternal effects. J Anim Sci. 2010;88:1276–1285. doi: 10.2527/jas.2009–2287.
6. Distl O. Mechanisms of regulation of litter size in pigs on the genome level. Reprod Domest Anim. 2007;42:10–16.
7. Robert S, Martineau GP. Effects of repeated cross-fosterings on preweaning behavior and growth performance of piglets and on maternal behavior of sows. J Anim Sci. 2001;79:88–93.
8. Milligan BN, Dewey CE, de Grau AF. Neonatal-piglet weight variation and its relation to pre-weaning mortality and weight gain on commercial farms. Prev Vet Med. 2002;56:119–127. doi: 10.1016/S0167–5877(02)00157–5.
9. Quiniou N, Dagorn J, Gaudré D. Variation of piglets’ birth weight and consequences on subsequent performance. Livest Prod Sci. 2002;78:63–70. doi: 10.1016/s0301–6226(02)00181–1.
10. Michiels J, De Vos M, Missotten J, Ovyn A, De Smet S, Van Ginneken C. Maturation of digestive function is retarded and plasma antioxidant capacity lowered in fully weaned low birth weight piglets. Br J Nutr. 2012;3:1–11.
11. Herpin P, Damon M, Le Dividich J. Development of thermoregulation and neonatal survival in pigs. Livest Prod Sci. 2002;78:25–45. doi: 10.1016/s0301–6226(02)00183–5.
12. Salmon H, Berri M, Gerdts V, Meurens F. Humoral and cellular factors of maternal immunity in swine. Dev Comp Immunol. 2009;33:384–393. doi: 10.1016/j.dci.2008.07.007.
13. Hansen JA, Nelssen JL, Goodband RD, Weeden TL. Evaluation of animal protein supplements in diets of early-weaned pigs. J Anim Sci. 1993;71:1853–1862.
14.Coffey RD, Cromwell GL. The impact of environment and antimicrobial agents on the growth response of early-weaned pigs to spray-dried porcine plasma. J Anim Sci. 1995;73:2532–2539.
15. Normantiene T, Zukaite V, Biziulevicius GA. Passive antibody therapy revisited in light of the increasing antibiotic resistance: serum prepared within a farm reduces mortality of dystrophic neonate piglets. Rev Med Vet. 2000;151:105–108.
16. Owusu-Asiedu A, Nyachoti CM, Baidoo SK, Marquardt RR, Yang X. Response of early-weaned pigs to an enterotoxigenic Escherichia coli (K88) challenge when fed diets containing spray-dried porcine plasma or pea protein isolate plus egg yolk antibody. J Anim Sci. 2003;81:1781–1789.
17. Pierce JL, Cromwell GL, Lindemann MD, Russell LE, Weaver EM. Effects of spray-dried animal plasma and immunoglobulins on performance of early weaned pigs. J Anim Sci. 2005;83:2876–2885.
18. Peek SF, Semrad S, McGuirk SM, Riseberg A, Slack JA, Marques F, Coombs D, Lien L, Keuler N, Darien BJ. Prognostic value of clinicopathological variables obtained at admission and effect of antiendotoxin plasma on survival in septic and critically ill foals. J Vet Intern Med. 2006;20:569–574.
19. Spier SJ, Lavole JP, Cullor JS, Smith BP, Snyer JR, Sischo WM. Protection against clinical endotoxemia in horses by using plasma containing antibody to an Rc mutant E. coli (J5). Circ Shock. 1989;28:235–248.
20. Tyler-McGowan CM, Hodgson JL, Hodgson DR. Failure of passive transfer in foals: Incidence and outcome on four studs in New South Wales. Aus Vet J. 1997;75:56–59.
21. Bourne F J, Curtis J. The transfer of immunoglobulins IgG, IgA and IgM from serum to colostrum and milk in the sow. Immunology. 1973;24:157–162.
22. Klobasa F, Butler JE. Absolute and relative concentrations of immunoglobulins G, M and A, and albumin in the lacteal secretions of sows of different lactation numbers. Am J Vet Res. 1987;48:176–182.
23. Rooke JA, Bland IM. The acquisition of passive immunity in the new-born piglet. Livest Prod Sci. 2002;78:13–23. doi: 10.1016/s0301–6226(02)00182–3.
24. Cutler RS, Fahy VA, Cronin GM, Spicer EM. Preweaning mortality. In: Straw BE, Zimmerman JJ, D’Allaire S, Taylor DJ, eds. 9th ed. Diseases of Swine. Ames, Iowa: Iowa State University Press; 2006: 993–1009.
